Pollutants Control Chemistry
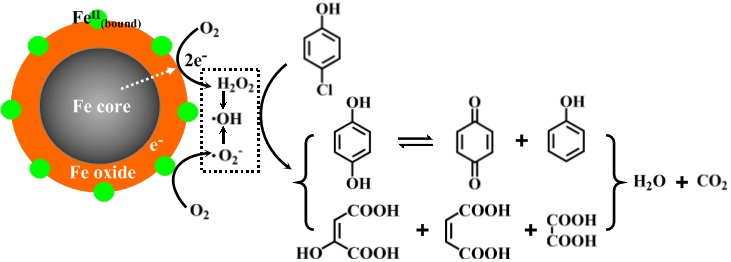
The rapid economic development brings more and more serious environmental problems, which is mainly caused by the chemical pollutants. The major purpose of pollutants control chemisty is study the chemical issues in the mechanisms and technologies related to pollutants control and remediation,aiming to develop economic and efficient pollutants control and remediation techniques, and provide theoretical basis for clean production process. Our group mainly focuses on the develop of pollutant control technology with environment benign iron and molecular oxygen, solving the key scientific problems faced for the nanoscale zero-valent iron reactivity tuning and the molecular oxygen activation. Recently, we revealed the core-shell structure dependent property of nanoscale zero-valent iron on the pollutant removal with molecular oxygen activation, and proposed a novel molecular oxygen activation mechanism to explain this phenomenon (please see the following figure). We are now seeking for some ligands to improve the nanoscale zero-valent iron reactivity,and clarifying the interface reaction of nanoscale zero-valent iron and the generation of reactive oxygen species.
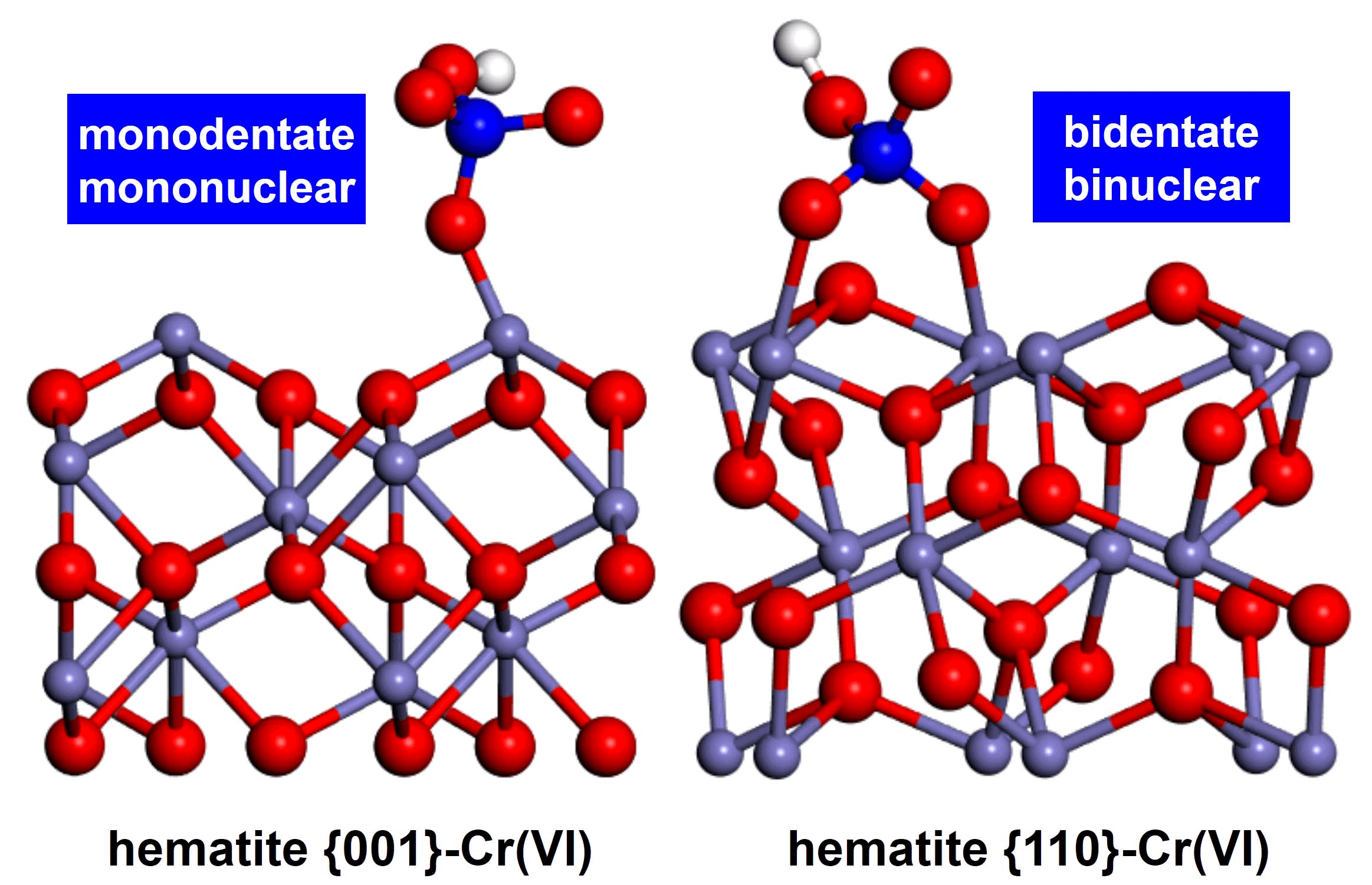
Iron is the fourth most abundant transition metal in the earth's crust, widely existsin aerosol, soil components, natural water systems, airborne minerals dust, animals and plants. Because of its ubiquity and redox property iron plays important roles in the bio-geochemical cycles and chemical evolution processes of living organisms. Asiron cycle is one of the most important and multifunctional bio-geochemicalcycles, the study of iron cycle and environmental effects becomes the international research frontier and hot topic, and also the key point to clarify the abiotic geochemical cycles of red clay soil. The solution to solve iron cycle issue lies in the understanding of adsorption/desorption, redox process and electron transfer on the suface/interface of iron oxide and iron (oxy)hydroxide natural minerals, as well as their environmental effects at an atomic level. Recently, we synthesized hematite nanocrystals with different facet exposure, and utilized synchrotron−based Cr K–edge extended X−ray absorption fine structure (EXAFS) spectroscopy, in situ attenuated total reflectance Fourier transform infrared (ATR−FTIR) spectroscopy, in combination with density−functional theory (DFT) calculation, to systematically investigated the adsorption/desorption,redox process and electron transfer on the different facets of hematite nanocrystals (please see the following figure), aiming to clarify environmental effects of hematite at an atomic level, understand the complex geochemical cycles of red clay soil and the intrinsic relationship between iron cycle and pollutant transport and transfer, and then realize environmental remediationand recovery with iron cycle manipulation.