Photocatalysis
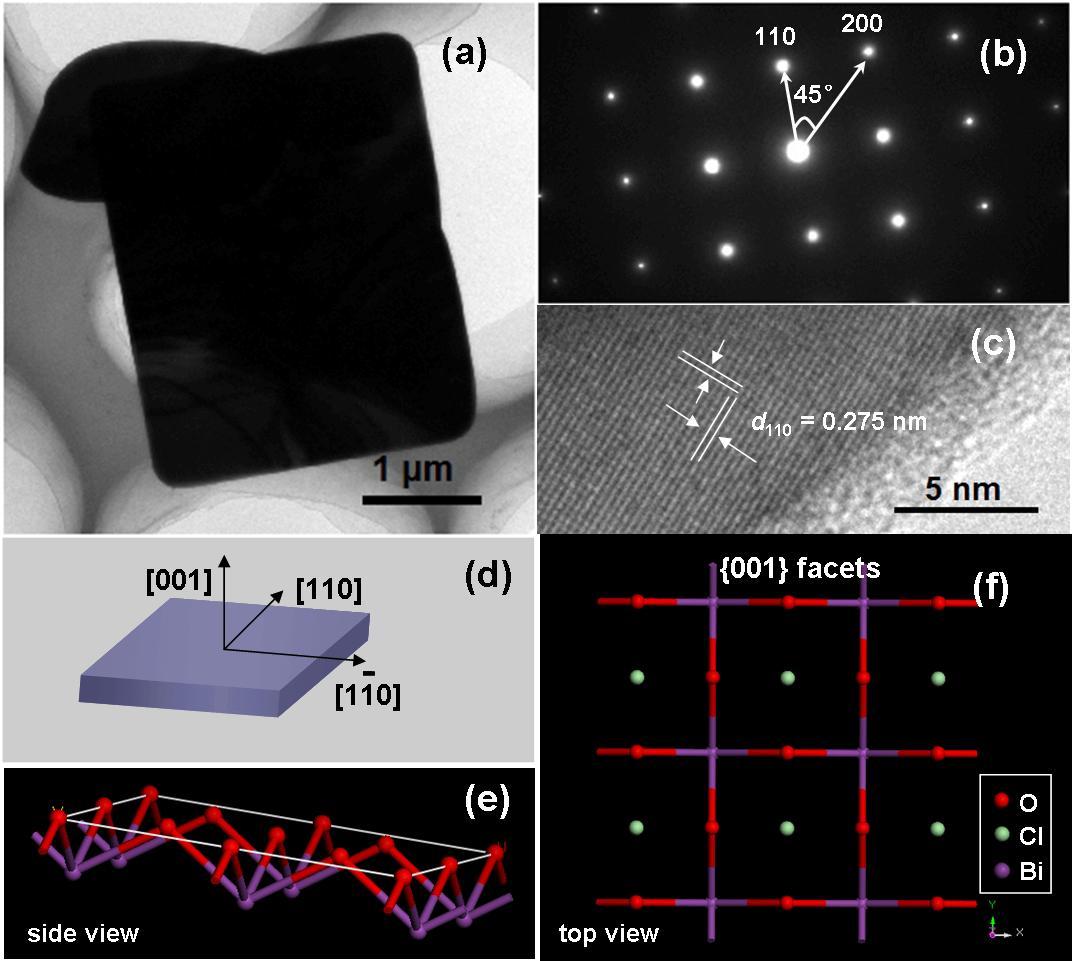
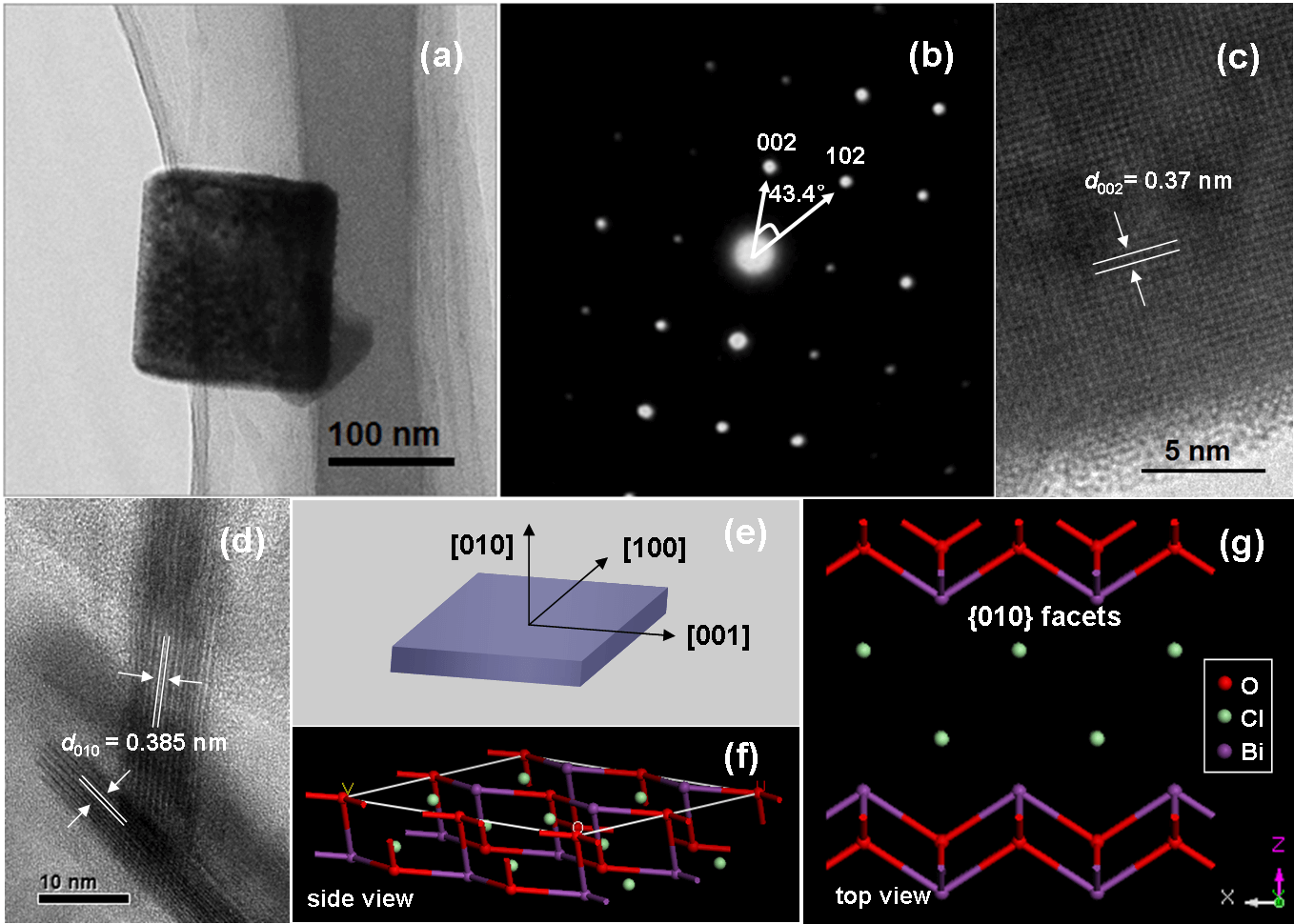
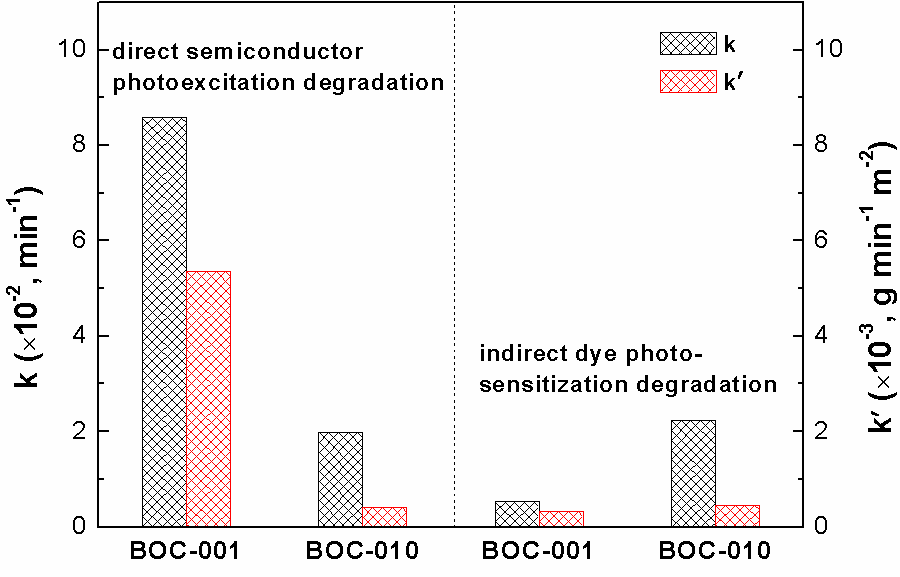
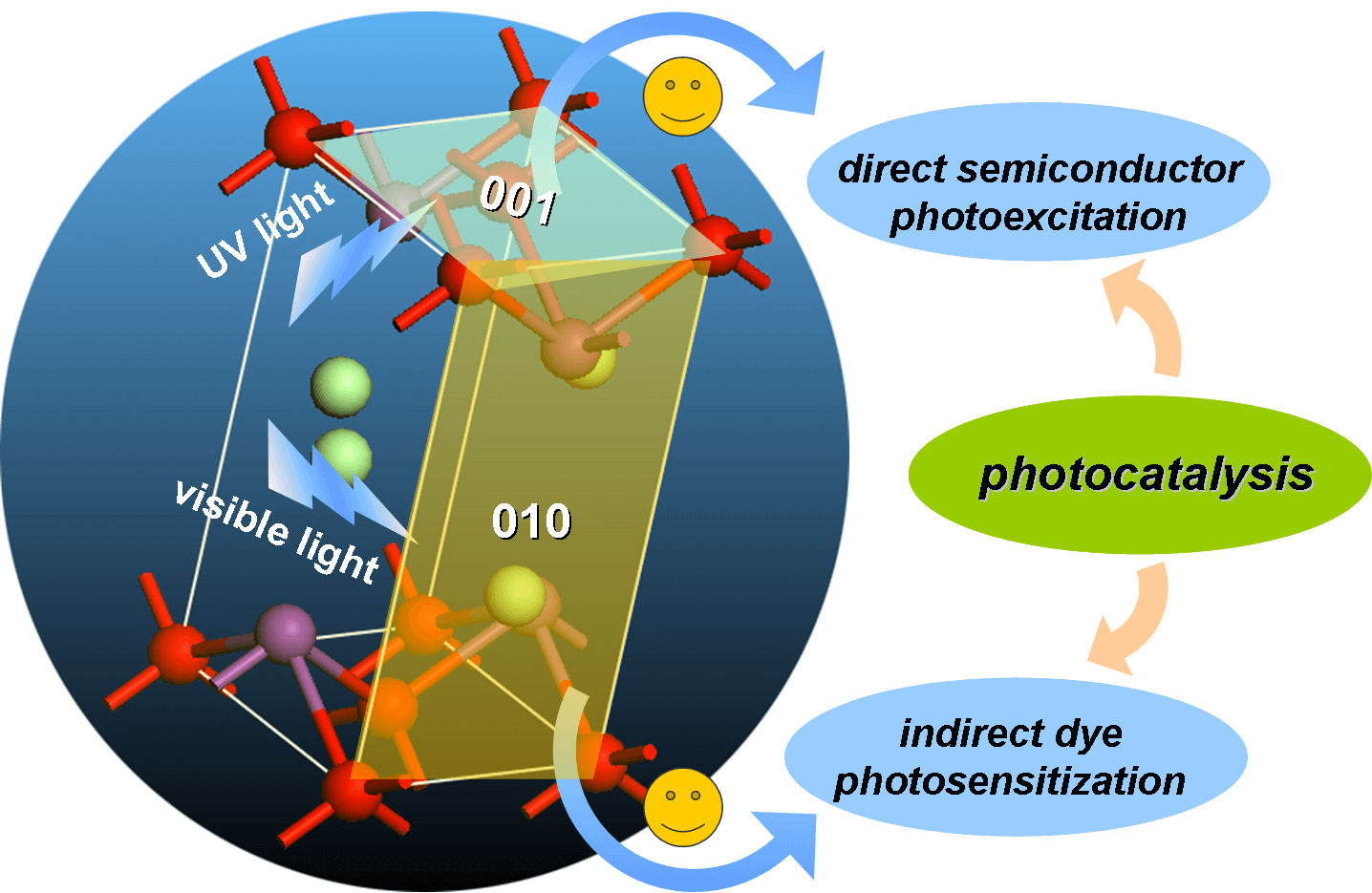
Photocatalysis can utilize solar energy to produce ·OH to oxidize organic pollutants, and also split water to generate H2, thus exhibit attractive application potentials to solve energy crisis and environmental pollution. However, its low quantum efficiency restricts its wide applications in the energy and environmental fields. How to improve the photocatalytic reaction efficiency is always the hot topic of photocatalysis. Our group aims to develop new low-cost strategies to prepare high surface area visible light photocatalysts, clarify their molecular oxygen activation, photocarriers separation and structure-performance relationship, and thus improve their pollutant degradation efficiency via molecular oxygen activation under ambient temperature and pressure with utilization of solar energy. For instance, we recently selectively synthesized BiOCl single-crystalline nanosheets with exposed {001} and {010} facets, and found that the resulting BiOCl single-crystalline nanosheets with exposed {001} facets exhibited higher activity on direct semiconductor photoexcitation pollutant degradation under UV light, but the counterpart with exposed {010} facets possessed superior activity on indirect dye photosensitization degradation under visible light (pleasesee the following figure).These findings will deepen our understanding of molecular oxygen activation on surface structures in photocatalytic reactions and allowus to sensitively manipulate the reaction processes.
Nitrogen is needed by all living organisms to build proteins, nuclei acids and many other biomolecules.Though constituting about 78% of the Earth’s atmosphere, nitrogen, in its molecular form, is unusable to most organisms because of its strong non-polar N-N covalent triple bond towards dissociation, negative electron affinity, high ionization energy and so on. Thus, the industrial fixation of N2 to NH3 through the classical Haber-Bosch process has to be conducted under drastic conditions (15~25 MPa, 300~550 oC) in the presence of iron-based catalyst to overcome the kinetic limitation, while consumes 1~2% of the world’s annual energy supply and produces 2.3 ton of fossil-derived CO2 per year. In view of the fossil fuels shortage and global climate change,nitrogen fixation through less energy-demanding process is therefore achallenging and long-term goal. In contrast, nature uses nitrogenase in vivo to catalytically reduce N2 to NH3 under mild conditions, because its MoFe-cofactor can activate N2. Our group employed BiOBr nanosheets of oxygen vacancies as the catalyst, and demonstrated that the designed catalytic oxygen vacancies of BiOBr nanosheets on the exposed {001} facets, with the availability of localized electrons for π-back donation, have the ability to activate the adsorbed N2, which can thus be efficiently reduced to NH3 by the interfacial electrons transferred from the excited BiOBr nanosheets (please see the following figure). This study might open up a new vista to fix atmospheric N2 to NH3 through the less energy-demanding photochemical process.Although photocatalytic reduction is unlikely to replace the Haber-Bosch process at present, this study might open up a new vista to fix atmospheric N2 to NH3 through less energy-demanding photochemical process.


