JACS |上交大张礼知&幺艳彩联合深圳大学杨波:单原子配位调控实现高效反向氢溢流与电催化脱氯

▲ 第一作者: Qian Zheng, Hengyue Xu
通讯作者:Yancai Yao Bo Yang Lizhi Zhang
通讯单位:上海交通大学 深圳大学
全文速览
本研究系统揭示了Rh单原子在氧化钛载体上的配位环境(Rh–O配位数从3到5)对反向氢溢出(RHS)过程的关键调控作用。四配位Rh₁O₄结构通过优化Rh的d带中心位置(–1.66 eV),实现近乎中性的氢吸附自由能(ΔG_H* = +0.08 eV),显著降低RHS能垒至+0.55 eV,从而在电化学脱氯反应中表现出卓越性能,4-氯酚降解速率常数高达4.65 h⁻¹,分别为五配位和三配位结构的4倍和29倍。
研究背景
反向氢溢出(RHS)是电化学氢化中的关键过程,涉及原子氢(H)从载体(如TiO₂)向金属活性位点的迁移。单原子催化剂(SACs)因其原子级分散和近100%的H利用效率而受到关注,但其配位环境与RHS活性之间的构效关系尚不明确。理解配位几何与电子结构(如d带中心、氧化态)对H*吸附与迁移的调控机制,是设计高效RHS电催化剂的关键。
本文要点
-
通过DFT计算揭示Rh–O配位数对d带中心、Bader电荷和H*吸附能的调控规律。
-
成功合成并表征了具有明确Rh–O配位(3, 4, 5)的Rh单原子催化剂(Rh₁O₃, Rh₁O₄, Rh₁O₅)。
-
四配位Rh₁O₄在RHS过程中表现出最优的H*迁移动力学和最低的能垒。
-
在4-氯酚(4-CP)电化学脱氯中,Rh₁O₄的降解速率常数为4.65 h⁻¹,法拉第效率最高,且具有良好的稳定性和pH适应性。
-
通过H*淬灭、EPR和KIE实验证实RHS是4-CP降解的决速步骤。
图片解析
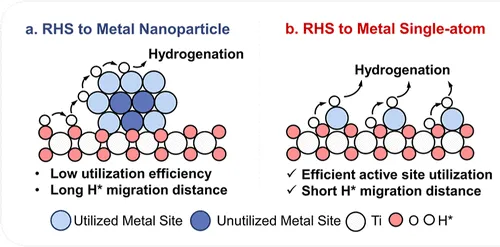
示意图比较纳米颗粒与单原子催化剂中H*迁移路径,突出单原子RHS的原子级距离优势
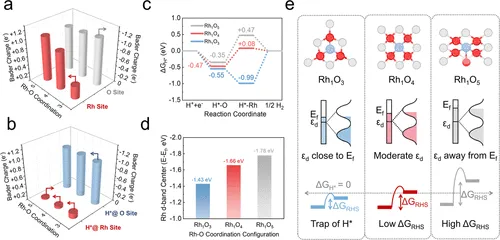
DFT计算结果:Bader电荷随配位数增加而升高(Rh₁O₃: +0.31 e⁻ → Rh₁O₅: +1.07 e⁻)
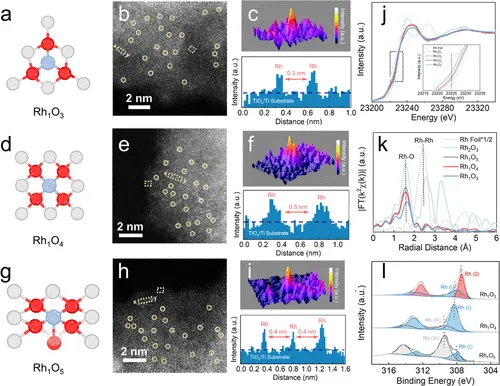
HAADF-STEM图像确认Rh单原子分散
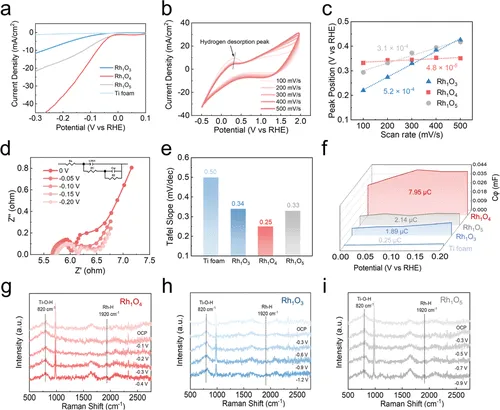
LSV显示Rh₁O₄起始电位最正(–0.07 V vs RHE)
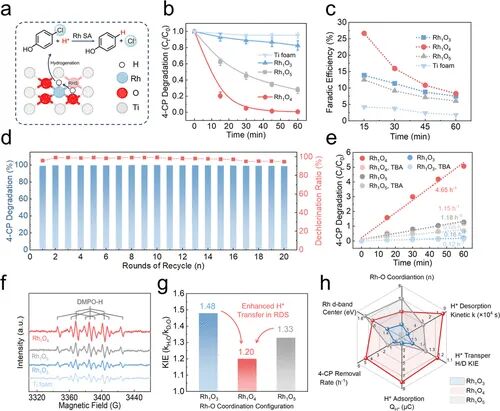
Figure 5. 4-CP degradation performance and mechanism investigation. (a) Schematic illustration of H* generation, transfer, and 4-CP hydrogenation process. (b) 4-CP degradation on Rh1O3, Rh1O4, Rh1O5, and Ti foam. (c) Faradaic efficiency of 4-CP hydrogenation on Rh1O3, Rh1O4, and Rh1O5. (d) Stability experiment for 4-CP hydrogenation on Rh1O4 in 20 rounds of electrolysis. (e) 4-CP degradation on Rh1O3, Rh1O4, and Rh1O5 with 0.1 mol/L of TBA. (f) EPR spectra. (g) KIE plot of 4-CP degradation rate constant ratio (KIE = kH2O/kD2O). h. Schematic illustration of Rh–O coordination-derived differences in single-atom RHS capacity and 4-CP hydrogenation activity.
性能验证
4-CP降解性能:Rh₁O₄在45分钟内实现99%脱氯
结论
Rh单原子的配位环境通过调控d带中心位置,直接影响H*吸附能与RHS能垒。四配位Rh₁O₄结构在电子结构与反应动力学上达到最优平衡,显著提升RHS效率与电化学脱氯性能。本研究为单原子电催化剂的配位工程提供了理论依据与设计原则。
意义和展望
本研究首次建立了Rh单原子配位数与RHS动力学之间的定量构效关系,为高效氢化催化剂的设计提供了新思路。未来可拓展至其他金属单原子体系(如Pt、Co)及更复杂的氢化反应(如CO₂还原、硝基化合物加氢),并探索在实际水体修复中的工程应用。
文献信息
Regulation of Rh Single-Atom Coordination for Enhanced Reverse Hydrogen Spillover and Efficient Electrochemical Dechlorination Journal of the American Chemical Society, 2025.
DOI: https://pubs.acs.org/doi/10.1021/jacs.5c18184
转自https://mp.weixin.qq.com/s/pXnm_FL5HA4yUPwbAyQJXA